Coating corrosion resistance inspection --- sulfur dioxide industrial gas corrosion test
The main corrosion in urban or industrial atmospheric environments is caused by the emission of sulfur dioxide into the atmosphere by the industrial sector, so SO2The corrosion test is a commonly used and standardized test; It is a test method to simulate the environmental conditions of gas pollution in industrial cities and carry out artificial accelerated corrosion of coatings. It uses a certain concentration of sulfur dioxide gas to corrode the coating at a certain temperature and relative humidity. The test results are very close to the actual corrosion of the coating in the industrial atmosphere, and are also about the same as the results of the CASS method and the corrosion tone method. This method is suitable for: Corrosion resistance test of Cu_ Ni_ Cr layer or Cu_ Sn alloy Cr layer on steel matrix. It can also be used to determine the cracks of the Cr layer of Cu_Sn alloys, the pan point of the zn_ Cu alloy layer, and the bubbling and eruption of the chromium layer on the copper or brass matrix.
1. Methodological principle
The corrosion test of the coating is carried out with a certain concentration of sulfur dioxide gas at a certain temperature and relative humidity, and the corrosion degree of the coating is checked and evaluated after a certain period of time.
2. Test the equipment
There are various special sulfur dioxide gas corrosion Test Chambers, as shown in Figure 11_3_5.
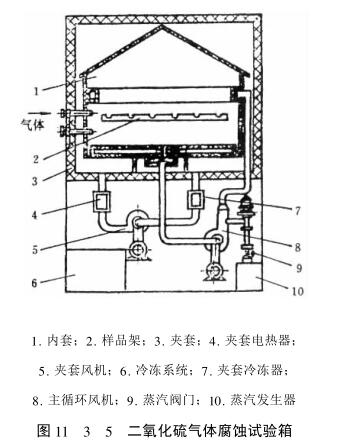
3. Test conditions
(1) sulfur dioxide concentration 0.1 (vt%);
(2) Temperature: 35~50°C;
(3) Relative humidity 95% ~ 100%;
(4) Keep the constant temperature and humidity within 8 hours of the test cycle, open the lid of the box within 16 hours, and place it at room temperature. Take a 24-hour cycle. (Related Instruments: Constant Temperature and Humidity Test Chamber)
4. Test method
(1) Sampling shall be carried out according to the technical requirements of the product.
(2) The surface of the tested coating should be removed with xylene alcohol solution, etc., but the coating should be prevented from being damaged.
(3) The clean specimen is strung with plastic wire, hung on a glass rod, and placed in the Test Chamber. The distance between the sample and the sample, and between the sample and the wall of the box should not be less than 2~4cm.
(4) Seal the lid of the box, and introduce the specified concentration of sulfur dioxide gas at the specified temperature and relative humidity, and keep the concentration constant throughout the test.
(5) After the test, the sample should not be dried by any method when taken out of the box, but should be dried by natural air or artificially until dry, and then the corrosion degree of the coating should be checked and evaluated.
5. Evaluation of test results
The inspection method and evaluation of the corrosion status of the sample can be carried out with reference to the inspection and evaluation method of the cAsses method or the corrosion paste method.
6 Preparation and analysis of sulfur dioxide gas
SO2 gas for testing, except for directly purchased cylinder S02 gas, is generally prepared by the following methods.
1) It is prepared by reaction of sodium sulfite and sulfuric acid
Na2S03 deca H2S04 -- S02 deca Na2S04 deca H20
During the test, a beaker is placed in the box, a certain amount of sodium sulfite is added to the cup, and then the lid of the box is closed and the temperature is started. When the temperature is close to the test temperature (about 3°C), the required sulfuric acid is added from the separating funnel outside the box to produce sulfur dioxide gas.
The amount of sodium sulfite and sulfuric acid required in the above reaction can be calculated as follows:
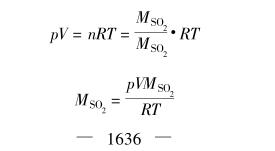

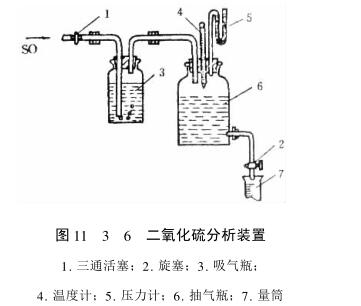
During the analysis, 300mL of distilled water and 5mL of 11 fixed powder indicator solution were added to the mouth and gas cylinder, and several clear iodine solutions were added until they were light blue, and then a total of 10mL of 0.1mo]/L standard concentration iodine solution was cleared from the cleaning tube, and one end of the mouth and the glass tube of the gas cylinder was connected with the Test Chamber with a rubber tube (introduced into the solution of the mouth and the gas cylinder), and the other end was connected with the pumping cylinder with a rubber tube. During operation, the piston that communicates with the Test Chamber is first closed, and the outlet piston of the extraction cylinder filled with water is opened, and a small amount of water leaks out at this time. If the device does not leak, the water will not flow out. Then put a graduated cylinder at the outlet of the gas cylinder, slowly open the piston connected with the Test Chamber, make the S02 pass through the inlet and the gas cylinder, maintain the gas flow speed, and keep moving the mouth and the cylinder until the blue color of the solution in the mouth and the cylinder disappears, and then stop the gas conduction. Record the volume of water flowing out of the cylinder and the negative pressure, temperature and atmospheric pressure in the cylinder. At this point, the volume of water flowing out of the cylinder is s02The volume of the gas. Calculate the concentration of S02 in the gas:

- 1Artificial climate accelerated weathering test and natural aging conversion
- 2Purpose and method of durability testing of building materials
- 3Cyclic corrosion test and its test procedures
- 4Coil coatings aging test
- 5The principle and application of salt spray corrosion Test Chamber, the operation and selection method
- 6What are the artificial simulated salt spray corrosion test methods?
- 7Cyclic Corrosion Testing and Applicable Standards
- 8Principle of cyclic corrosion test
- 9What is Automotive Cyclic Corrosion Test CCT?
Mark
-
HZAOC LSO2-100 SO2 corrosion Test Chamber$ 15123.00
-
HZAOC SO2-300 SO2 corrosion Test Chamber$ 15358.00
-
HZAOC SO2-250 SO2 corrosion Test Chamber$ 15358.00







