Determination of film corrosion rate by polarization method
The electrochemical method of determining the corrosion rate using the polarization curve of the corrosion resistance of the test paint film has hardly been used; Ordinary corrosion processes on metals are indistinguishable, so the polarization method should be considered very promising.
Figure 189 shows the schematic diagram of the instrument for measuring the corrosion contact velocity by the polarization method (according to the Akimov method). The test method is as follows: two standard test pieces 1 and 2 of the same material are immersed in the electrolyte. Each electrode is polarized by the external current power supply 3, so that one becomes an anode and the other becomes a cathode. The size of the voltage used can be changed by the resistor 4 (related instrument: resistance meter). Potential can be measured by potentiometer 5 . The current generated between the electrodes can be measured by the sensitive ammeter 6 (to measure the change of the potential of the coating film, a Gansu electrode can be used). In order to make the test results more accurate, it is better to place the test pieces close to each other.

In Fig. 190 is shown the equipment used to bring the test piece close to the device according to the Akimov method.
A polarization curve showing how the electrode potential should change upon polarization is plotted on FIG. 191 . The right side of the curve (cathode) shows the potential change at one electrode, and the left side (anode) shows the potential at the other electrode. Point E. Corresponds to the electrode potential when not polarized by an external power source. (Related instrument: conductivity meter)
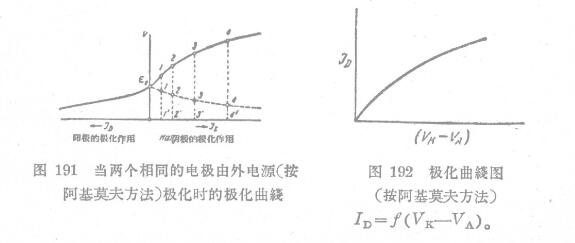
The polarization curve of the anode is also indicated in the cathodic part on the right. At this time, l-1, 2-2, 3-3 and other potential differences (e K -s a ) correspond to a certain current density "Dl', 2', 3', etc. According to the measurements made Polarization curve (electrode potential difference-current intensity, Figure 192).
If the curve rises sharply upwards, that is, a large current intensity corresponds to a small potential difference, the corrosion contact speed is very high. The slanted end indicates that the contact velocity is small in this case.
- 1ISO 9227: How to check for corrosion of salt spray enclosures?
- 2Coating corrosion resistance performance inspection method - neutral salt spray test
- 3Corrosive influencing factors of ship painting in soil
- 4Determination of Corrosion Resistance of Long-lasting Anticorrosive Coatings
- 5Chemical and Corrosion Resistance Testing of Films
- 6Coating mass testing - corrosion resistance test
- 7Determination of film corrosion rate by Electrochemistry
- 8Determination of Corrosion Resistance of Films by Volume Test and HNNJIK
- 9Determination of corrosion resistance of film films