A paper to understand the determination of optical rotation
The phenomenon of optical rotation is a unique property of optically active substances (such as organic compounds containing asymmetric carbon atoms) to rotate in the plane of polarized light, which is of great scientific significance. In the chemical, pharmaceutical and food industries, the optical activity of optical rotation substances (such as sugars, amino acids, drugs, etc.) is closely related to their molecular structure, so optical rotation measurement has become a key means to identify the purity of substances, distinguish optical isomers and analyze the three-dimensional structure. For example, in drug discovery, polarimetric assays can be used to verify the optical purity of chiral drugs to ensure their biological activity; In the food industry, polarimetric measurement is often used for the concentration analysis of carbohydrates. In addition, optical rotation provides important data support for stereochemical research, such as the spatial configuration of complex molecules through optical rotation dispersion (ORD) and circular dichroism (CD) techniques. Therefore, polarimetric measurement is not only a basic method for the characterization of substances, but also an important tool to promote scientific research and technical application in related fields.
Optical rotation phenomenon and specific optical rotation
1. Definition and characteristics of optical molecules
Optical rotation substances are substances that can rotate the vibrating surface of polarized light. These substances usually have chiral molecules, i.e., their molecular structure cannot coincide with their mirror images. Optical rotation substances exhibit specific optical rotation in optically active solvents and can be used for substance identification and concentration determination.
2. The relationship between asymmetric carbon atoms and optical rotation
The presence of asymmetric carbon atoms (chiral centers) in the molecule is one of the key factors for optical rotation. The asymmetric carbon atom is attached to four different groups, which makes the molecule asymmetrical, which leads to the rotation of polarized light. For example:
Sucrose (C₁₂H₂₂O₁₁): contains multiple chiral carbons and exhibits right-handed (d-type) properties.
Menthol (C₁₀H₂₀O): The chiral center in which it has optical rotation and its different enantiomers, such as l-menthol and d-menthol, exhibit opposite optical rotation directions.
3. The physical mechanism of polarized light and optical rotation
Polarized light is a light wave that is fixed in a plane in the direction of electric field vibration. When polarized light passes through a photo-rotating substance, its vibrational direction rotates, forming a photo-rotating phenomenon. This is due to the fact that the circularly polarized light of the optical rotation molecule has a different refractive index (i.e., optical path difference) for left- and right-handed light, resulting in the overall rotation of the linearly polarized light.
Interpret the optical rotation process
Incident light - ordinary light passes through a polarizer and becomes polarized light in a single direction of vibration.
Different components of polarized light are refracted by optical rotation solutions – optical molecules produce different degrees of refraction, causing the vibrating surface of the light to rotate.
The direction of polarization shifts after rotation depending on the specific rotation, concentration, and path length of the substance.
4. Mathematical expression of specific rotation ([α]).
Specific rotation is defined as the optical rotation per unit concentration and per unit pathlength, and is often used to characterize the optical rotation characteristics of pure substances. It is calculated as follows:
Solution system formula:
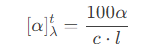
Pure liquid system formula:

Multiple effects of temperature (t), wavelength (λ), and solvent on optical rotation
5. Factors affecting optical rotation
Temperature (t): Increasing temperature may alter the intermolecular forces and affect the optical rotation.
Wavelength (λ): Optical rotation usually varies with wavelength, and short-wavelength light (e.g., 365nm) usually causes greater optical rotation.
Solvent effects: Different solvents may affect the dissolved state of the solute and thus the optical rotation measurement.
The structure and principle of the optical rotation instrument
Conventional disc polarimeters, such as the WXG model, are mainly composed of a sodium light source, a polarizer, a polarizer, a semi-shade piece, and a polarimetric tube. During the measurement, the polarized light rotates after passing through the optical rotation solution, and the operator adjusts the brightness of the field of view by rotating the polarizer to read the optical rotation angle. The semi-shade structure improves the measurement contrast, but there is human error, and the accuracy can be optimized by adding the filter.
The modern automatic indication polarimeter adopts an optical mechatronics design, integrating a Faraday coil modulator, a photomultiplier tube and a servo motor closed-loop control system to achieve automatic polarimeter measurement. Signal modulation (50Hz/100Hz) reduces ambient light interference, differential angle compensation improves measurement stability, and digital direct reading avoids human error for more accurate results.
Compared with traditional polarimeters, automatic polarimeters have the advantages of high precision, fast measurement and automatic data processing, which are suitable for quality control in pharmaceutical, food, chemical and other industries. Its automated measurement system reduces human error and improves repeatability and reliability, making it ideal for efficient testing of large sample batches.
Optical Rotation Measurement Experimental Operation Specification
Sample preparation and instrument calibration
In optical rotation measurement, the choice of solvent is crucial, and different solvents (e.g., water, ethanol) can affect the direction of the optical rotation and the measurement accuracy. In general, a solvent that adequately dissolves the sample without interfering with the polarimetric measurement is selected, and the specific rotation correction of the solvent is taken into account to ensure the accuracy of the measurement results.
The sample concentration and layer thickness need to be strictly controlled to ensure the stability of the optical rotation angle. Standardized assay tubes (e.g., 10 cm) are often used to reduce errors and ensure that the solution is of the right concentration to avoid multimolecular scattering or optical distortion due to excessive concentrations. In addition, avoid the use of coverslips with mechanical stress to prevent stress birefringence from affecting the measurement accuracy.
Instrument calibration is an important step in polarimetric measurements, and is typically benchmarked using a zero-point calibration and a standard polarimetric substance such as a quartz sheet. While traditional polarimeters require manual adjustment of the zero point, automatic polarimeters utilize a servo system for precise correction, combined with digital compensation technology to reduce systematic errors, thereby improving measurement repeatability and accuracy.
Key Steps
Before the polarimetric measurement, a zero correction is performed first, i.e. a solvent blank test is used to ensure that the instrument baseline is accurate. The steps involve injecting a pure solvent into the tube, placing it in a polarimeter to measure the readings, and adjusting the instrument to the zero point. The test is repeated several times to verify the stability of the zero point reading to ensure the accuracy of subsequent sample measurements.
Determining the direction of the optical rotation is one of the key steps to avoid the misjudgment of the ± 180° optical rotation angle. Concentration dilution can usually be used, i.e., the trend of the rotation angle of different concentrations of samples can be measured to ensure that the direction of the rotation is consistent. If the rotation angle is still close to ±180° after dilution, the assay parameters need to be remeasured or adjusted. At the same time, the polarimeter three-point field adjustment method can be combined to further confirm the direction of optical rotation by observing the changes of light and darkness to reduce the error.
Throughout the measurement process, the thickness of the liquid layer should be kept consistent, the tilt of the measuring tube or the interference of air bubbles should be avoided, and the influence of environmental factors (e.g., temperature, humidity) should be noted. For automatic polarimeters, digital compensation and differential angle calculations can be used to optimize measurement accuracy and improve repeatability and reliability.
Error control and precautions
In optical rotation measurements, temperature control is crucial because temperature fluctuations affect the density of the solution and the movement of molecules, which in turn can change the angle of rotation. Laboratories often require temperature fluctuations to be controlled within ±0.5°C, and stable measurement conditions can be achieved by means of a thermostatic Water Bath or ambient temperature control equipment. For more temperature-sensitive samples, the temperature at the time of measurement should be recorded and corrected using the specific rotation correction formula.
For turbid samples, suspended particles or impurities can cause light scattering, making the measurement results unstable and therefore requiring pretreatment. Common methods include centrifugation (sedimentation of impurities at high speeds) and filtration (e.g., 0.45 μm microporous membrane to remove particulates). For difficult-to-clarify samples, a suitable solvent can be selected to dissolve or dilute to reduce turbidity and improve measurement accuracy.



