The role, performance and characteristics of anti-corrosion coating
(1) The role of anti-corrosion coating
The anti-corrosion coating forms a coating after the surface of the substrate to be coated is cured to prevent corrosion of the substrate. Its functions are as follows.
1 screen factory effect
The screen function of the coating is to isolate the substrate from the external environment so as to avoid its corrosion. According to the principle of electrochemical corrosion, the corrosion of the metal under the coating requires the existence of water, oxygen, and high particles, as well as the passage of high particles, blocking the penetration of water, oxygen and high particles, so as to prevent metal corrosion. However, very large screens are impossible, and any coating has some degree of permeability.
2. Corrosion inhibition
The chemical anti-rust pigment contained in the coating, in the presence of water, decomposes the corrosion inhibitor high ion from the pigment, thereby causing anodic polarization, or cathodic polarization, or both cathode and anode polarization, and inhibits the progress of corrosion. Corrosion inhibitors can make up for the deficiency of the barrier effect, and the barrier effect can prevent the loss of corrosion inhibitors, and the two complement each other.
3. Electrochemical action
The metal powder that can become a sacrificial anode to the base metal is added to the paint, and the electric connection angle between the metal powder and the metal powder and the base can reach the degree, which can protect the base from corrosion, such as zinc-rich primer for steel Protect.
(2) Requirements for the performance of anti-corrosion coatings
Only when the basic properties of anti-corrosion coating, stability to medium, adhesion (including wet adhesion) and mechanical strength meet the requirements can it realize its protective effect on the base metal.
1. Penetration resistance
Penetration resistance is the most basic requirement for anti-corrosion coatings. As a kind of high polymer film, the coating can block the penetration of water, oxygen and high particles to different degrees, so as to play an anti-corrosion role.
The penetration of water into the coating is considered to be through the process of adsorption, dissolution, diffusion and capillary attraction. The former two are related to the polar groups and soluble components contained in the paint-based polymer, and the latter two are related to the mobility of polymer chains, the porosity of the coating and the amount of leaching. Soluble components and leachables include water molecule monomers, retained solvents, foreign pollutants and polymer degradation products. The porosity of the coating is determined by the pinholes of the coating and the pores between the polymer molecules and inside the macromolecules. Pinholes are caused by unsatisfactory construction. The porosity is inherent in the coating, which depends on the molecular structure, crosslink density and arrangement state of the polymer. Penetration of the coating cannot therefore be absolutely avoided. High polymer membranes with regular structure, tight arrangement, and only a small amount of hydrophilic groups have low water permeability (such as chlorinated rubber, polyvinylidene chloride, etc.). According to the experimental data, the relationship between the water vapor permeability and the corrosion resistance of the coating is shown in Figure 4_2_1.
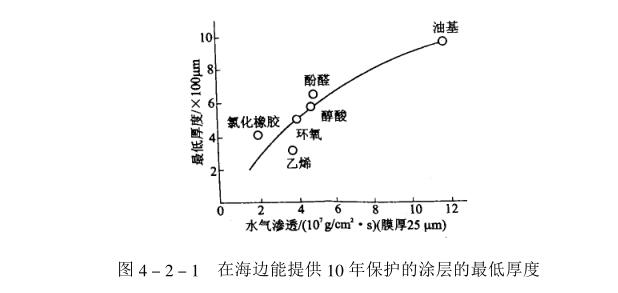
It can be seen from Figure 4_2_1 that the coating with low water vapor permeability needs a low coating thickness to provide the same protection period.
The addition of pigments can improve the penetration resistance of the coating. The pigment particles are impervious to water and can fill the pores of the tubes, prolonging the path of penetration into the substrate. When the amount of pigment in the coating is less than the critical pigment volume fraction, water penetrates through the base material between the pigment particles; but when the pigment amount is greater than the critical pigment volume fraction, water diffuses faster through the gaps between the pigment particles. For paint films containing pigments, the measured water vapor transmission rate and water transmission rate are different because water can accumulate at the interface between the pigment and the base material, and the water will pass through the capillary flow of the small pores of the paint film to penetrate. enter. So some paint films are less resistant to water than they are to water vapor. The thicker the coating, the lower the water permeability, so the heavy-duty anti-corrosion coatings are all thick film type (High Build).
For a paint film with no defects and small holes, water travels through the free volume holes in the paint film, but the actual paint film inevitably has small holes, and the pore diameter is larger than the holes, so that water, gas, and ions can easily pass through. enter. If multi-layer coating is applied, the defect hole will not extend to the base plate, and the penetration needs to pass through the free volume cavity, which retards the penetration and corrosion.
The permeability of gas is much smaller than that of water (see Table 4_2_2), and the permeability of gas is not directly related to that of water.


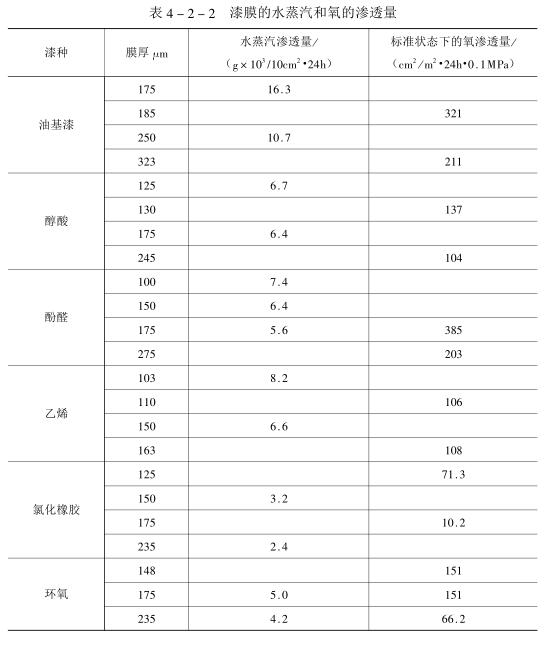
In the process of electrochemical corrosion, high electrons are needed to participate in the reaction, and the resistance of the coating can block the passage of high electrons and retard the occurrence of corrosion. The mechanism of the penetration of hypertrons in the coating is similar to that of water, and the presence of water is required for hypertrons and their movement. Gaozi penetrates the paint film much slower than water and oxygen, thus retarding the corrosion process. After most paint films are soaked in water, the residues contained in them are decomposed, which makes the paint film negatively charged, thus selectively attracting the high ion to penetrate into the paint film.
2. Stability to corrosive media
The stability of the anti-corrosion coating to the corrosive medium means that it is neither chemically decomposed by the medium nor has a harmful reaction with the medium, nor is it physically dissolved or swelled by the medium. From the perspective of medium corrosion resistance, the carbon chain polymer is better than the hetero chain, the hydrogen atoms on the carbon chain are replaced by fluorine and chlorine atoms, the saturation is high, and the polarity is less than the double bond. More polar groups are better.
3 Adhesion and Wet Adhesion
For the coating to effectively protect the substrate, it needs to adhere firmly to the substrate during the service life. In addition to the reactive primer, the adhesion of the coating mainly depends on the physical attraction between molecules (also known as the secondary valence bond force), which includes orientation force, induction force, dispersion force (called van der Waals force above) and hydrogen bond force. Among them, the hydrogen bond force is the strongest, but this kind of attraction can only be produced in the high molecular range, so the primer should have good wettability, so that the paint can fully contact the substrate. Table 4_2_3 lists the nature of the intermolecular forces.
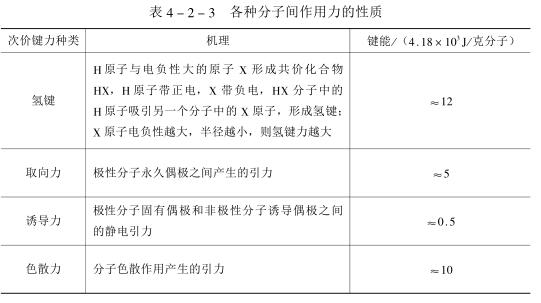
During the use of the coating, there are two main factors affecting the adhesion:
其一,涂层一金属界面上水的积聚。水对金属的亲和力大于一般高聚物对金属的亲和力,故水能插入其中间,取代高聚物的吸附。界面上的水可能来自施工时金属表面原来吸附的水,影响涂层原始附着强度,也可能在使用过程中,水由涂层表面渗入。
其二,内应力的积累。随着涂层固化后期的溶剂挥发,使用过程中的进一步交联和水分子物质析出等因素,使涂层体积收缩而形成内应力。使用中冷热、干湿的循环交替,涂层与基体的涨缩系数不同会导致界面产生反复的相对位移,形成破坏性应力。内应力大于附着力,涂层会脱开;内应力小于附着力而大于内聚力,涂层会开裂。据测定因体积收缩而形成的内应力可达9.8x103kP。
高聚物的结构与内应力形成有关。较柔软的涂层能通过分子构象变化消除内应力,高交联的刚性涂层则不行。片状、纤维状颜料也能降低涂层的内应力,但代价是颜料与高聚物间微观开裂。
所请“湿附着力”是指涂装于物体上的漆膜在水中浸泡一段时间后的附着力。这是近年来将漆膜附着力与耐腐蚀性相联系的新认识。Funk等人认为,若漆膜湿附着力差,透过漆膜到达钢铁表面的水分子与钢铁表面发生作用,就可以顶替掉原来的漆膜和钢铁表面的作用而形成水层,而透过漆膜的02便可以溶解于漆膜下的水。由于有02和H20,钢铁便有了发生腐蚀的条件。腐蚀一旦发生,此时水成为盐的溶液,于是有渗透压产生。在渗透压作用下,H20和02可非常迅速地通过漆膜,此时的漆膜相当于半透膜,漆膜的附着受到进一步破坏,导致与钢铁表面脱高(气泡因之生成)。另一方面,腐蚀发生时,体系中有0H一高子产生,它可使一些易水解的基团水解,如酯基,使漆膜失去应有的机械物理性能,从而失去保护钢铁的作用。
按照湿附着力理论,涂料具有良好防腐蚀性的基础是漆膜在钢铁表面上有极住的湿附着力。如果漆膜的透水性、透氧性低,膜下溶解高子少,渗透压低,可以延长湿附着力降低的时间,则涂膜的保护期将得到延长。
4.机械性能
涂层的机械性能指标有硬度、柔韧性、耐冲击性、耐磨性等,机械性能可以综合地反映作为粘弹体的涂层在受外力时产生变形的大小。
The mechanical properties are related to the glass transition temperature Tg of the polymer, and Tg is determined by the structure of the polymer molecule. The service temperature of the coating should be higher than the Tg of the base material. If the Tg is higher than the service temperature, a plasticizer needs to be added. Durable anti-corrosion coatings should have little change in Tg during use.
The mechanical properties of the coating depend on the relationship between the mechanical stress it bears and the strain distribution generated inside the polymer structure. The stress-strain characteristics of the coating are related to the type of polymer, the type and concentration of pigments and fillers. When the volume fraction of pigment and filler is almost lower than the critical range of pigment volume fraction A, the tensile strength of the coating film increases and the elongation decreases with the increase of pigment A value. Low elongation and low tensile strength of the coating film indicate that the coating film is hard and brittle, indicating that it is not durable; low elongation and high tensile strength indicate that the coating film is hard and tough; high elongation and low tensile strength The strength indicates that it is a soft elastic film; both values are high for a strong elastic film.
- 1Analysis of Surface Defects of Explosion Holes and Fisheye-Anticorrosive Coatings
- 2What is an anti-corrosion coating?
- 3Failure of anti-corrosion coatings
高瑾 米琪 - 《《防腐蚀涂料与涂装》》
- 4Double top coating: how it works, how to apply it
Shivananda Prabhu
- 5Preparation method of polyaniline anti-corrosion coating
- 6The main advantages of metal thermal spray coating or hot dip mirror zinc and organic coating
- 7Introduction to anti-corrosion mechanism of coating
- 8Construction method of heavy anti-corrosion painting brushing
- 9Determination of Corrosion Resistance of Long-lasting Anticorrosive Coatings