Two forms of metal corrosion: uniform corrosion and localized corrosion
The consequence of metal corrosion is nothing more than the damage caused by metal. Usually, the damage mode of metal corrosion can be divided into two types: uniform corrosion and localized corrosion.
The so-called uniform corrosion is that the corrosion occurs evenly on the entire metal surface, and the whole will be gradually corroded, including various properties of the metal, so the harm it brings is not too serious, at least gradually.
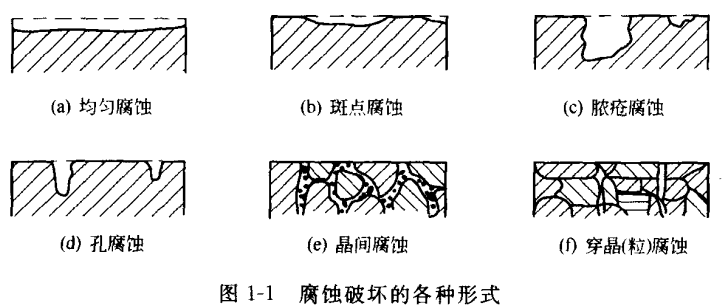
Localized corrosion is the local area where the corrosion is mainly concentrated in the metal. Because the distribution, depth and development of these corrosions are very uneven, often when the overall metal is still quite intact, the local corrosion is quite serious, which will lead to serious accidents or disasters, so it is very harmful. Local corrosion generally has the following forms.
①Pitting corrosion Corrosion is distributed on the metal surface like spots, occupying a large area, but not very deep, see Figure 1-1 (b).
②Corrosion of abscess The metal is corroded and damaged like a sore on a human body, and the damaged part is deeper and larger, as shown in Figure 1-1 (c).
③ Pore corrosion (also known as pitting corrosion) becomes some small and deep round holes in some parts of the metal, and sometimes even perforation occurs, see Figure 1-1 (d).
④ Intergranular corrosion This corrosion occurs on the edge of the metal crystal. When a metal suffers from intergranular corrosion, the bonding force between its grains is significantly reduced, and the internal structure becomes very loose, so that the mechanical strength is greatly reduced, as shown in Figure 1-1 (e).
⑤ Transgranular (grain) corrosion is a kind of local corrosion that destroys along the maximum stress line, and its characteristic is that the corrosion often penetrates the metal grain body. Stress corrosion cracking is also one of them, see Figure l-1 (f).
⑥ In the selective corrosion of multi-element alloys, if one component is dissolved into the corrosive medium, another component will be enriched on the surface of the alloy, which will change the properties of the alloy. Corrosion in this case is also called decomposition corrosion. For example, brass dezincification, aluminum bronze dealuminization, copper-nickel alloy de-nickeling, etc. all belong to this type of corrosion.