Electroless Plating and Coating Systems Overview
Overview of Electroless Plating
In electroless plating, the metal plating is formed due to the chemical reaction between the reagents in solution and the reducing agent present in the metal ions . The metallic phase that occurs in this reaction can be obtained either in the bulk of the solution or as a precipitate as a film on the solid surface. The localization of a chemical process on a particular surface requires that the surface need to act as a catalyst. If the reduction product (metal) of the catalyst itself is guaranteed, in this case it is in principle that the coating thickness is infinite. This self-catalytic reaction constitutes the essence of the practical process of electroless plating. Therefore, these electroplating processes are also sometimes referred to as autocatalytic.
Electroless plating may include metal plating techniques in which metal is due to the decomposition reaction of a specific compound; for example, depositing a layer of aluminum. Decomposition of complex aluminum hydrides in organic solvents. However, these methods are difficult to operate , and they have little practical significance.
Broadly speaking, electroless plating also includes other metal deposition processes. Where no external current is used, such as immersion and contact plating methods. Another negative (active) metal acts as a reducing agent. However, this approach is limited. Uses: They are not suitable for metallization of dielectric materials and non-catalytic reactions . Therefore, they are not generally classified as electroless plating.
Electroless plating is currently widely used in the surface modification of various materials, such as insulators , semiconductors, and metals. Among the methods of using metal paint, only the electroplating technology exceeds the volume , and it is almost equivalent to vacuum metallization.
The electroless plating method has several advantages over similar electrochemical methods. details as follows:
1. The coating can be deposited on non-conductive materials (on almost any surface, stable in chemical plating solution).
2. The thickness of the coating is more uniform, regardless of the shape of the coating.
3. The deposition is simple, and it is sufficient to soak (pre-treat) the product in electroless plating.
4. Coatings with unique mechanical, magnetic and chemical properties can be obtained.
Compared with electroplating technology, the application of electroless plating is limited by two aspects. Factors: (a) It's more expensive because the reducing agent costs more than an equivalent amount of current . (b) In most of the solutions , the strength is low because the metal deposition rate is limited by the reduction of metal ions.
Coating system
To ensure the chemical reduction of metal ions in solution, the solution needs to contain a sufficiently strong and active reducing agent, that is, it needs to have a sufficiently negative redox potential. The easier the reduction of metal ions , the greater the amount of available reducing agent. Since there are only autocatalytic reduction reactions, the systems that can be successfully used to deposit coatings, electroless plating of Me-Red (a reducing agent for metals) and are practical are not very large (see Table 28.1).
Currently known electroless plating methods can be used to deposit 12 different metals, including metals belonging to the iron, copper, and platinum groups (well-known catalysts for various reactions) as well as tin and lead (only one solution has been published for post-deposition By). Although the deposition of chromium and cadmium coatings is described in the patent literature, autocatalytic reduction is not achieved in these cases. Coatings can only be deposited on some metals by immersion plating.
In some widely used processes, metal deposition is accompanied by reduction precipitation of reagent decomposition products - boron and phosphorus, etc., to obtain the respective alloys. It is not difficult to deposit two or more metals at once; the electroless plating method is known to deposit over 50 alloys of different qualitative compositions, mainly based on nickel, cobalt, and copper.
Most of the reducing agents used in electroless plating are hydrogen compounds, where H is related to phosphorus, nitrogen and carbon. It is in the reactions of these compounds that significant catalysis is possible because these reactions proceed slowly in the absence of catalysts.
A stronger reducer is obtained when effective autocatalysis - phosphoric acid - is applied. In the absence of a catalyst, reducing agents are inert and do not even react with strong oxidizing agents.
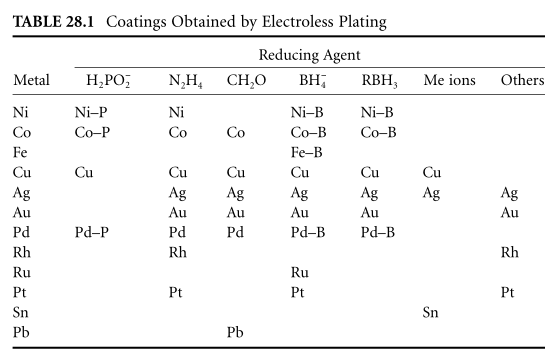
Few catalysts are suitable (such as nickel, cobalt and palladium), but they provide the highest catalytic process without reducing the rate of solution volume . Other reducers are also more versatile, for example by using sodium borohydride we can deposit almost all metal coatings mentioned. The reducing power of hydrogen compounds increases with the pH of the solution. For this reason, most electroless plating solutions are alkaline.
Such simple reducing agents as variable valence metal ions (Fe 2+ , Cr 2+ , and Ti 3+ ) are generally not suitable for the deposition of coatings because non-catalytic reduction tends to occur. Recently, the state of autocatalytic deposition of tin and silver as reducing agents such as metal complexes Sn(OH) 4 2 −, Co(NH3) 6 2− has been established .
The technique of chemical reduction of certain metals (silver, gold, Cu) was considered a long time ago. In the nineteenth century, but after it became popular, Brenner discovered (1945) a very effective electroless nickel plating process using sodium hypophosphite. At the time, the term "electroless plating" was coined.
- 1How to detect surface gloss of platings?
- 2What are the practical applications of electroless plating?
- 3Electroless Plating Solutions
- 4What is electroless plating?
- 5The performance of electroless deposited metal coatings
- 6Several different electroless plating
- 7Four basic technical parameters of electroless plating solution
- 8Deflection method inspection method of coating internal stress
- 9Type characteristics, inspection methods and test conditions for coating surface defects