Several different electroless plating
Electroless plating is divided into the following types:
copper deposition
Although different reducing agents are used to deposit copper plating, only formaldehyde copper plating solutions are of practical interest. Formaldehyde undergoes copper ion-catalyzed reduction at room temperature in alkaline solution (pH = 11–14); here, copper ions need to be bound to a complex. Electroless copper plating solution Cu2+ aptamers of polyols (polyhydric alcohols, hydroxyacid anions) and compounds with tertiary amine groups and hydroxyl groups (hydroxylamine, EDTA, and others). In practice, tartaric acid, EDTA, and ethylenediamine (ethylenediamine) are commonly used.
During the copper plating process, with the progress of the main reduction reaction,
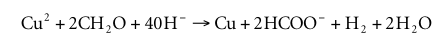
Formaldehyde is consumed in the Cannizzaro reaction, and a total of 3 to 6 moles of CH 2 O is deposited for 1 mole of Cu consumption. In copper electroplating, multiple bases are used including the Cannizzaro reaction. The consumption of OH can be determined according to the following formula (amount of substance in moles):
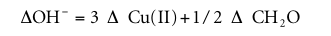
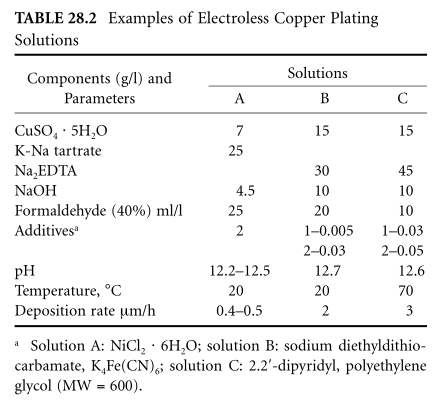
Various formulations of copper plating solutions, which are completely stable and suitable for long-term development protocols (eg, Solution B, Table 28.2), have been developed. Three types of electroless copper baths have been distinguished in the literature: (i) solutions with low deposition rates (0.5 to 1 μm/h), suitable for copper layer deposition; (b) solutions with 4 μm deposition rates of 5 m/h hours (i.e. exhibit high catalytic effect); and (c) for high toughness and high copper coating deposition solutions (eg, Table 28.2 for C solution). All of these solutions basically have the same ingredients: they differ mainly in their additives. Furthermore, additive processes have been used to produce high toughness coatings for printed circuit boards at high temperatures (40°C) and relatively low copper deposition rates.
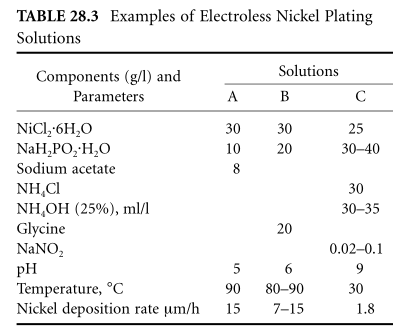
nickel plated
Electroless nickel plating, with sodium hypophosphite as the reducing agent, is a popular process. But autocatalytic reduction of phosphoric acid by nickel ions occurs in both acidic and basic solutions. In a stable solution with high coating quality, the deposition rate may be as high as 20~25μm/h, but due to the formation of hydrogen ions in the reduction reaction, a higher temperature is required, about 90°C,
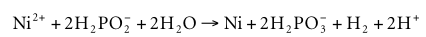
Solutions with high buffer capacity are necessary to ensure a stable process. For this, acetic acid, propionic acid, citric acid, lactic acid, glycolic acid, or glycine are added to the solution; these substances, along with buffering, can form nickel complexes. Required for binding NI 2+ ion complexes in alkaline solutions (here, in addition to citric acid, glycine, ammonia and phosphoric acid can be added); moreover, this binding is desirable in acid solutions, since free nickel ions form Reaction product compounds (eg, phosphate), precipitate out, hindering further use of the solution.
Additions of stable nickel solutions are not necessary for copper solutions, however, they are added to ensure long-life solution stability.
Phosphorus is always present in the coating when the reduction is done by phosphoric acid. Its amount (between 2 and 15 mass percent) depends on the pH, buffer capacity, ligand, and other parameters of the chemical solution.
Borohydride salts and their derivatives can also be used as reducing agents for electroless nickel plating solutions. And 60 to 90 ℃ temperature for the reduction of borohydride nickel ions, dimeth ylaminoborane (DMAB) makes Ni B coatings deposit a small amount of boron (0.5 ~ 1 mass%) at 30 to 40 ℃. Neutral and alkaline solutions Its composition is similar to that of sodium hypophosphite solution within the usable temperature range.
Cobalt, iron, tin plating
Cobalt-nickel deposits are similar, the same reducers (sodium hypophosphite, sodium borohydride, and their derivatives) are used, and the relationship between the reductions is similar. 14 The reduction of cobalt is difficult, and the deposition rate of cobalt is lower than that of nickel. It should be noted that the deposition of cobalt from acidic solution is difficult. The resulting cobalt and phosphorus coatings are of particular interest due to their magnetic properties.
Electroless iron plating is relatively difficult, and only one sufficiently efficient iron plating solution is known, in which Fe ions form a complex tartrate and NaBH4 acts as a moderator. In an alkaline solution (pH 12), an iron-boron coating (about 6% b) of about 2 μm/h was deposited at a temperature of 40 °C.
It is quite difficult to realize an autocatalytic TiN deposition process. A sufficiently efficient tin deposition method is based on tin(II) disproportionation in alkaline media. 15 In 1 to 5 million sodium hydroxide solution, at 80-90 ℃, a deposition rate of several microns per hour can be deposited.
Precious Metal Deposition
Electroless silver plating is the oldest electroless metal plating process, but currently lags behind nickel or copper plating in performance. An unstable disposable ammonia silver plating solution (containing glucose, tartaric acid, formaldehyde, etc.) is usually used. The coating thickness of this solution is not very large (<1μm). This unstable solution is more suitable for aerosol spray.
More efficient solutions for electroless silver plating have been developed using silver(I) complex aminoboranes or hydrazine as reducing agents: deposition rates of 3 to 4 μm/h at temperatures of 40 to 50 °C, in stabilizers These solutions are fairly stable in the presence of . Sufficiently stable electroless silver plating solutions can be obtained using metal ions such as cobalt(II) compounds as reducing agents.
Gold plating can be deposited with various reducing agents: however, the solutions are generally unstable. Sufficiently stable solutions have been developed using the stable gold cyanide complexes of sodium borohydride or dihydrogen as reducing agents. 16 At 70-80°C, the deposition rate reaches 5 point in the morning/h, and a gold coating with sufficient purity is obtained.
A thin layer of gold can be deposited on plastic by aerosol spray method, and hydrazine is used as a reducing agent to obtain a thicker coating (deposition rate up to 0.4 μm/min).
Palladium coatings are readily deposited using sodium hypophosphite as the reducing agent in alkaline solutions, where the PD 2+ ions are bound in a complex of ammonia, EDTA, or ethylenediamine. When palladium plating is at 40-50°C, the deposition rate of palladium-phosphorus (4-8 P) coating is in the range of 2-5 μm/h.
Platinum, ruthenium and rhodium coatings can be deposited using ammonium borohydride or hydrazine as reducing agents. The process rate in a stable solution is very low (0.5-2 μm/h).
metal alloy deposition
About 60 paints of different mass compositions containing two or more metals can be deposited. Metals such as copper, iron, zinc, tin, rhenium, tungsten, molybdenum, manganese, thallium and platinum group metals can be introduced into nickel and cobalt deposits, while nickel, cobalt, tin, zinc, cadmium, antimony, bismuth, lead and gold are introduced into the copper plating.
In chemically deposited metal alloys, the thermodynamic relationship is the same as that of alloys deposited by electroplating techniques. Obviously, it is difficult to introduce metals in the plating layer into hard-to-reduce metals such as chromium and manganese. Furthermore, in the case of chemical reduction, another factor of the metal - the catalytic performance - becomes apparent. Large amounts of additional metal can be added to nickel, copper, etc. platings only if the metal is catalyzed or at least inert to the oxidation of the reducer. The catalyst content may be as high as 100% in the metal, the catalytically inert metal may be as high as 50%, and the metal corrosion inhibitor may be as little as 10 to 20%. When less catalytically active metal is introduced, the deposition rate decreases.