Detailed steps for pH determination of solutions
Instruments and reagents
1. Instrument
(1) The pH measurement range of the pHS-2 acidity meter is 0~14, divided into 7 ranges, each with 2 pH units, and the accuracy is ±0.02pH/3pH. Its panel structure is shown in the figure.
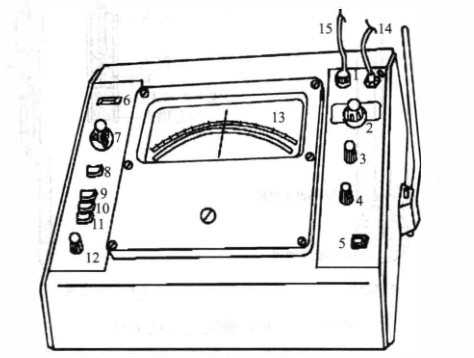
pHS-2 acidity meter panel structure
1 - electrode connector and socket; 2 - step switch; 3 - calibration regulator; 4 - positioning regulator; 5 - reading switch; 6 - indicator light; 7 - temperature compensation adjustment; 8 - power button; 9-pH Button; 10--10mV button; 11--mV button; 12 — set point regulator; 13 — indicator; 14 — connect glass electrode; 15 — connect Gancai electrode
(2) pH glass electrode
(3) Gandu electrode
2. Reagents
(1) Standard buffer solution czo·c with a pH of 4.00 Weigh 10.12 g of analytically pure potassium hydrogen phthalate (KHCsH 4 0 4 ) dried at 115°C for 2 hours , dissolve in CO 2 -free
Dilute in deionized water to 1000mL in a volumetric flask, mix well, and store in a plastic bottle (you can also use commercially available standard buffer solution reagents in bags, dissolve in water, and dilute as required).
(2) Standard buffer solution with pH 6.88 (20 °C)
Weighed 3.40 g of analytically pure potassium dihydrogen phosphate ( KH2PO4) and 3.55 g of analytically pure disodium hydrogen phosphate (Na2HPO4) dried at 11 °C for 2 hours, and dissolved them in CO2-free deionized water, Dilute to 1000mL in a container, mix well, and store in a plastic bottle.
(3) Standard buffer solution ( 20 °C) with a pH of 9.22 Weigh analytically pure Na2B40, 10H2O.81g, dissolve in CO2-free deionized water, dilute to 1000mL in a volumetric flask, and store in a plastic bottle . The above standard buffer solutions are usually stable for two months.

l - electrode lead; 2- insulator; 3 - saturated KCI solution; 4 - rubber cap; 5 - internal electrode; 6 - insulator
Method and principle
Insert the indicator electrode (glass electrode) and reference electrode (saturated Gansu electrode) into the solution to be tested to form a primary battery.

Under certain conditions (25 ℃), the electromotive force E of the battery is a linear function of pH: E = K+o. 059 pH
The pH of the solution to be tested can be obtained from the measured electromotive force. However, because the K value in the above formula is a constant related , it is difficult to obtain it by calculation. In actual work, when measuring the pH value of a solution, a standard buffer solution of known pH must used to calibrate the acidity meter. When calibrating, a standard solution close to the pH of the measured solution should be selected. It is usually calibrated with two standard buffer solutions of different pH (the error should be within 0.05pH unit). A calibrated pH meter measures the pH of water or other solutions directly.
4. Determination steps
1. Preheating and electrode installation
Turn on the power and warm up the instrument for half an hour. Press the pH button, and the indicator light will light up. The glass electrode is connected to the negative terminal socket, and the saturated Gancai electrode is connected to the positive terminal. Adjust the temperature compensation so that it indicates the temperature of the solution being measured.
2. Zero point adjustment
测量pH时, 将分挡开关指向 "6"' 调节零点调节器使指示表指针在 "l" (本仪器是以1作为零点)。
3. 校正
将量程分挡开关旋至“ 校正 ”位置, 调节校正调节器使指针在满刻度 "2.0" 处。 每次调节时需待半分钟使指针稳定。 如此重复上述操作至 校正好为止。然后将玻璃电极接头插入插口中, 量程分挡开关旋至"6"处。
4. 定位
在烧杯中倒入pH标准缓冲溶液, 将电极浸入溶液中, 将量程分档开关调至标准缓冲溶液pH 范酣内, 按下读数按键, 调定位调节旋钮, 使表头上的读数加上量程分挡开关上所指的读数之和正好等于标准缓冲溶液的pH。 必要时可重复校正和定位操作, 使其定位准确。
5. 测量
将电极上移, 用蒸熘水冲洗, 并用滤纸吸千电极表面的水滴,再插入待测液中。 转动分挡开关至 所测的量程范围, 按下读数开关,表头指示数值与分挡开关指示之和即为测量值。测量完毕后, 取出电极, 冲洗干净, 将甘采电极吸干, 套上侧口和下端的两个橡胶套, 放回电极盒中。 而玻璃电极仍继续浸泡在蒸熘水中, 关上电源。
五、 注意事项
1、由于水样的pH常常随空气中CO2等因素的改变而改变,因此采集水样后应立即测定。
2、标准缓冲溶液的pH随温度不同稍有差异。
3、用蒸熘水或去离子水冲洗电极时, 应当用滤纸吸去玻璃膜上的水分, 而不是擦拭电极。
4. When using Gancai electrode, care should be taken to detect whether the lower end is immersed in the saturated KCI solution. The lower end of the electrode is plugged with a ceramic core, which allows a small amount of KCI solution to seep out during measurement , but does not allow the measured liquid to flow in. Therefore, the rubber plug on the side port on the upper side of the electrode should be pulled out when using to ensure sufficient liquid level difference.
5. Before using the pH glass electrode, the spherical membrane part of the electrode should be soaked in distilled water for more than a day and night, so that the asymmetric potential becomes smaller and the zero potential value tends to be stable. During use, the electrode membrane should be prevented from being stained or surface worn. Due to the high internal resistance of the glass electrode, the use of electromagnetic stirring may cause electromagnetic interference, and the eddy current caused by stirring may cause fluctuations in . Therefore, electromagnetic stirring is generally not used when measuring pH with a glass electrode. The correct operation is to insert the electrode into the solution, shake the measuring cup by hand or start stirring to fully contact the electrode with the solution, then stop stirring to measure.
6. The pH meter is a high-impedance measuring instrument, which requires good insulation performance between the two connection points connecting the electrodes. Therefore, when in use, the instrument's negative terminal jack and electrode plug should kept dry and clean. After the measurement is completed, the connector should be inserted into the jack to prevent dust and moisture from entering.
- 1Principle and Application of Standard pH Meter
- 2Method for determining amino acid pH value
- 3Basic principle and application analysis of anti-fluorine pH meter
- 4Proper use and maintenance of pH meters
- 5Understanding and Addressing Common Challenges in the Use of pH Meters
- 6How to use pH meter
- 7Differences between three STIP-scans: pH Meter, Conductivity Meter, and Dissolved Oxygen Meter
- 8PH meter conversion of mv and ph value
- 9Common types and application differences of STIP-scan