Surface tension of paint rheology
Surface tension is defined as the excess force per unit length on a surface; it is considered positive if it acts in the direction of the contracting surface. The tendency of the 1,6 system to reduce the surface area is the result of excess surface energy due to the different environment in which surface atoms are exposed compared to bulk atoms. The surface tension of liquids and polymer melts can be measured by methods such as capillary, 1 Du Nuoy ring, 2,7 Wilhelmy plate, 3,8 and sag. 4,5 We will focus on two methods: the capillary height method and the pendant drop method.
The capillary height method is suitable for low-viscosity liquids because it takes a long time for the system to reach equilibrium for high-viscosity liquids. It has been reported that it takes up to 4 days for a polystyrene melt to reach equilibrium at 200 °C. 5 Figure 2.1 illustrates the capillary height method. In equilibrium, the force exerted on the perimeter of the meniscus due to surface tension needs to be balanced by the weight of the liquid column. Neglecting the weight of the liquid above the meniscus, an approximate equation can be written as:
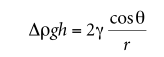
where Δρ is the density difference between the liquid and air, g is the gravitational constant, h is the height of the liquid column, γ is the surface tension, θ is the contact angle, and r is the radius of the capillary . In practice, it is difficult to accurately measure vertical contact angles with known and uniform radii. To more accurately determine surface tension, various methods can be used to calculate the weight of the liquid above the meniscus.
Table 2.1 Solventborne and Powder Coatings Application Methods and Markets
Figure 2.1 Capillary method
capillary. In practice, it is difficult to accurately measure vertical contact angles with known and uniform radii. To more accurately determine surface tension, various methods can be used to calculate the weight of the liquid above the meniscus.
The pendant drop method is a very versatile technique for measuring the surface tension of a liquid as well as the interfacial tension between two liquids. Andreas et al. 9 used this method to measure the surface tension of various organic liquids. Wu 10 and Roe 11 have widely used this method to measure the surface and interfacial tension of many polymer liquids and melts.
The experimental setup shown in Fig. 2.2 consists of a light source, a pendant drop cell and a syringe assembly in a constant temperature chamber and a photomicrograph. A typical shape of a pendant is shown in Figure 2.3. The surface tension of the liquid is
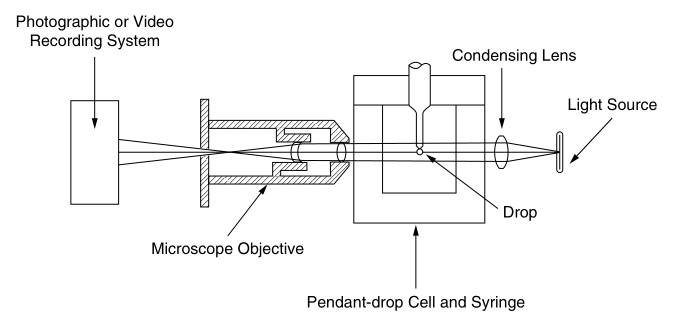
Figure 2.2 Experimental setup for the drape method
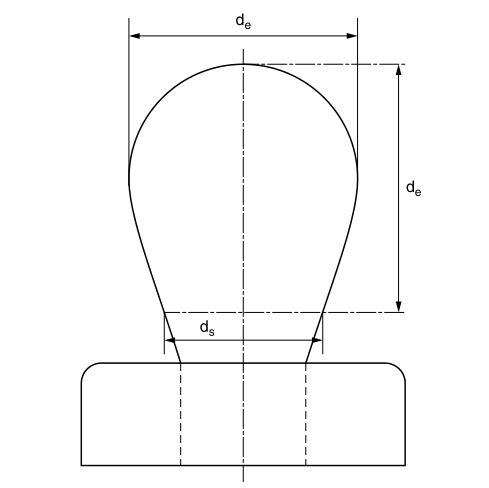
Figure 2.3 Typical fall profile
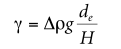
where de is the largest (equatorial) diameter of the pendant droplet and H is a correction factor that depends on the shape of the droplet; H is related to the measurable shape-dependent factor S defined as
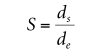
where ds is the diameter of the pendant drop in the selected plant and the distance from the apex of the pendant drop is de (see Figure 2.3). A table can be used to show the variation of 1/H with S. 12-14
Recently, many significant improvements have been made both in data acquisition and in the analysis of dangling curves. Photographic documentation and measurements of 1–17 pendants were replaced by direct digitization of video images. The ability to measure the entire drop profile has led to the development of new algorithms for drop profile analysis.
- 1Water surface tension measurement based on Electronic Balance
- 2Comparison of Liquid Surface/Interfacial Tension Testing Methods: Plate vs. Ring Method
- 3Selection guide for Surface Tensiometers
- 4Application principle and precautions of platinum ring Surface Tensiometer
- 5Coating surface tension and its effects
- 6Coating process surface tension factor
- 7Three commonly used detection methods for surface tension
- 8Ceramic ink-jet surface tension, viscosity and solutions
- 9Determination of interfacial tension and surface tension








