Determination of Color of Transparent Liquid by Platinum-Cobalt Colorimetry
1. Scope and description
This method specifies a method for evaluating the color of clear liquids in platinum diamond units. Platinum diamond unit is the solution color when 1L of barren solution contains 1mg of platinum in the form of chloroplatinate ion) and 2mg of cobalt(I) chloride hexahydrate. This method is suitable for clear liquids with a color similar to that of the Platinum Diamond Grade Standard. The platinum-cobalt grading method compares the color of a sample to a color standard and expresses the result in platinum-cobalt color units.
2. Instruments and Materials
(1) Spectrophotometer
It can measure the light transmittance at wavelengths of 430nm, 455nm, 480nm and 510nm, and the accuracy of the transmittance of the photometer should be 0.005 or more accurately.
(2) The optical path of the colorimetric cell is 10mm long.
(3) The structure of the Colorimeter allows the light to pass through the longitudinal axis of the colorimetric tube for comparison with vision, so that the white light transmitted or reflected from the white glass plate penetrates the colorimetric tube with equal intensity, There should be baffles at both ends of the Colorimeter to prevent light from penetrating into the colorimetric tube from the side.
(4) Colorimetric tubes are also commonly known as Nessler tubes (Nessler tubes), with a flat bottom and a capacity of 100mL. There are ground transparent glass stoppers. The color of the glass of each colorimetric tube should be the same. There are scale lines, and the height difference between the scale lines of each colorimetric tube shall not exceed 3mn.
3. Preparation of Color Standards
(1) Reagent
① Potassium chloroplatinate (KzPtCl6): analytically pure;
②Cobalt (I) chloride (CaCl2 6H2O); analytically pure ;
③ Hydrochloric acid (HCl): analytically pure, density 1.19g/mL;
④ Distilled water (H2O); GB/T 6682 Class II.
(2) Preparation
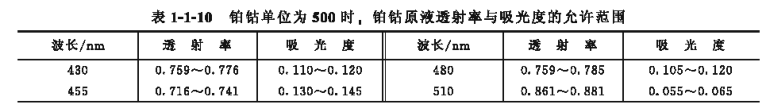
①Color stock solution (platinum-cobalt unit is 500) Weigh 1.245g of potassium chloroplatinate and 1.000g of cobalt(I) chloride, put them into a 400mL beaker, moisten with a small amount of distilled water, add 100mL of hydrochloric acid, and heat It dissolves to obtain a solution of . After the solution is cooled, transfer it to a 1000mL volumetric flask, dilute to the mark with distilled water, and spread evenly. Put the above color stock solution into the colorimetric cell, and measure it with a Spectrophotometer , if the transmittance meets the requirements in Table 1-1-10
The specified range of transmittance means that the solution has a color stock solution with a platinum cobalt unit of 500.
② Platinum-cobalt standard colorimetric solution
According to the range of requirements listed in Table 1-1-11, measure the platinum-cobalt stock solution into a colorimetric tube, dilute to 100mL with distilled water, and shake well. Then cover the colorimetric tube with a lid, seal it with shellac paint or a mixture of paraffin and rosin, and mark the platinum-cobalt unit on the colorimetric tube respectively.
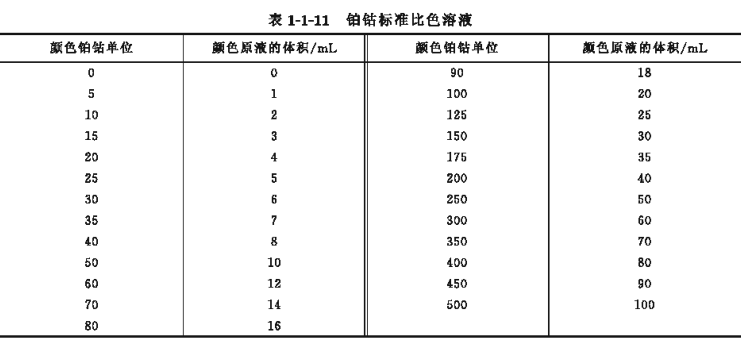
Store the sealed color stock solution in a dark place, and its color is stable for 1 year. If the platinum-cobalt standard colorimetric solution is stored in a dark place, it can be stable for half a year, but it is better to use the freshly prepared solution.
4. Measurement method
(1) Pour the sample into a colorimetric tube up to the mark line. If the sample is turbid or has visible impurities, it should be filtered first to obtain a transparent solution.
(2) Then close the lid and put it on the Colorimeter, observe it visually and compare it with the standard colorimetric solution until the color reaches a platinum-cobalt standard colorimetric solution that is closer.
5. Result presentation
(1) The color of the sample crystal is expressed by the number of platinum-cobalt units of a standard colorimetric solution that is closer to the color of the sample. If the color is between two standard colorimetric solutions, it is expressed by the number of the darker one.
(2) If the color characteristic of the sample crystal is different from that of the standard colorimetric solution, so that an exact comparison cannot be made, record the color unit that can obtain an approximate grade according to item (1), and explain the observed color, or record For "different hue".
6. Reference standard
National standard GB/T 9282 "Assessing the color of transparent liquids by platinum-zirconium grades".