How are pigment flocculation and sedimentation formed?
Our practice has proved that most inorganic pigments are hydrophilic, so they are easily accepted by water. Let's use an example of an inorganic pigment to explain how sedimentation occurs: when a polar (hydrophilic ) pigment (such as titanium dioxide) is mixed with water ( a polar liquid), oleic acid (a Surfactants with polar and non-polar groups ) and benzene ( a non-polar liquid ) , a situation like Figure 21-8 will occur: after the hydrophilic pigment is added to the water, in the container A solid settlement (sediment ) will form at the bottom . After the titanium dioxide is completely wetted by water (because the titanium dioxide has a greater attraction to water than itself), sedimentation occurs due to particle-to-particle contact. In this case, water aids in the settling process.
The situation is exactly the opposite when hydrophilic titanium dioxide is added to hydrophobic benzene. At this time, the original contact state between the titanium dioxide particles is still maintained, because non-polar benzene has no force to destroy the polar-polar attachment between adjacent pigment particles. As a result, the titanium dioxide particles form a flocculated structure - a mechanical network of vegetable and pine bonds, which can be maintained for a long time in a static state. Therefore, there is only very little pigment settling.
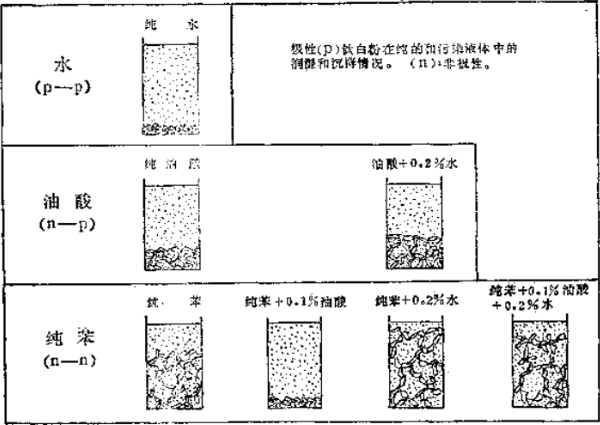
Figure 21-8 Flocculation and sedimentation of titanium dioxide in water, oleic acid and benzene in pure liquid and polluted liquid
The case of adding titanium dioxide to oleic acid represents a mixed dispersion condition ( that is, factors of water and benzene). The fatty (fat) part (lipophilic ) of oleic acid works at one end and the carboxyl part ( hydrophilic ) works at the other end, resulting in a significant loose sediment.
When a small portion of oleic acid was added to a dispersion of titanium dioxide in benzene, the dramatic effect on settling was evident. At this time, the characteristics of the sedimentation are completely changed from non-sedimentation (flocculation) to solid sedimentation, due to the nature of oleic acid. When the polar-nonpolar oleic acid is introduced into the titanium dioxide-benzene system, this surfactant will try to balance the hydrophobicity and hydrophilicity. The polar carboxyl group in oleic acid is attracted to the polar titanium dioxide surface, and once in contact, it adheres firmly. The fat ( fat ) part of oleic acid extends to the non-polar benzene region. Note that this effect occurs only at very small amounts of oleic acid, and only superficially. Since the orientation layer of oleic acid prevents any polarity-to-polarity contact of the closest titanium dioxide particles, a network structure cannot be formed, thereby preventing the titanium mortar powder particles from settling and forming a solid sediment (precipitate) at the bottom of the container. Phenomenon.
If a small amount of water is added to oleic acid or rice, the degree of flocculation of titanium dioxide will be increased, and flocculation will be caused because water can enhance hydrophilicity.
In the last case in Figure 21-8, where oleic acid and water are added to a dispersion of titanium dioxide and benzene, we can see that there is an interesting competition for the surface position of the titanium dioxide particles. This is due to the effect of surface activity on water.
The interconnected network structure formed by the flocculation of pigment particles is relatively weak, but there is enough force to stop the phenomenon of gravity pulling the particles down, thereby preventing their sedimentation. Conversely, deflocculated particles will settle into lumps ( unless something is done to prevent it). Understandably, flocculation and sinking are opposites. In fact, the sedimentation release of pigments is often measured by the degree of deflocculation of the assay system.
It should be noted that it is also very important to distinguish between the proportion of settlement and the degree of (later ) settlement. Generally speaking, the flocculated pigment particles settle faster than the deflocculated pigment particles, but the sedimentation ( precipitation ) is looser and can be redispersed, while the deflocculation pigment particles settle slower, but the sedimentation (precipitation) More solid, and not easy to disperse. The reason why the flocculated pigment settles quickly is simple because its particles are large and hard, which is easily explained by Stoke's law (large particles settle faster than small particles). However, the settling of these flocculated particle clusters will soon stop, because the flocculated aggregates can be cross-linked to form a network structure, thereby preventing further deformation (sedimentation).
- 1Importance and determination method of paraffin resistance of pigments
- 2How to determine pigment lightfastness
- 3How to assess the dispersion of pigments?
- 4Pigment acid alkalinity test
- 5Definition of pigment PVC formula, use of pigment
- 6What are the four major categories of coatings?
- 7How to increase the redispersion effect of pigment surface area?
- 8Pigment related ASTM testing standards
- 9Difference between pigment and dyestuff