Principles and Comparison of Three Preparation Methods of Enzyme Membrane
(1) Covalent binding method
For covalently bound enzyme molecules, the presence of covalently bonded binding sites on the membrane surface is a prerequisite. These binding sites are provided by functional functional groups carried by the membrane itself or introduced later. Various functional functional groups link enzyme molecules and membranes in the form of covalent bonds. In the process of immobilization, the membrane is often immersed in different enzyme solutions to complete the filtration. The process depends on covalently bound chemical reactions, sometimes with the help of an external energy source. For example photoligation, using light to increase the energy to induce a covalent reaction. A typical example is the conversion of azido functional groups into highly reactive nitrogen functional groups by UV light irradiation43. Sometimes in order to enhance the effect of immobilization, the distance between the enzyme molecule and the membrane is often increased, such as introducing a spacer arm. This is because direct immobilization of enzyme molecules on the membrane can lead to changes in the conformation of the enzyme molecules or reduction of active sites. The longer the molecular chain of the spacer arm, the more the immobilized enzyme molecule resembles the free enzyme molecule when it participates in the catalysis of chemical reactions. However, this does not mean that the introduction of long spacer arms can obtain high residual enzyme activity. The length of the spacer arm plays a small role in comparing the chemical bond reaction process and the membrane bulk properties. Although the short spacer is difficult to ensure good immobilized enzyme activity, the stability of the enzyme molecule has increased by an order of magnitude, because the functional groups involved in immobilization in the short spacer are just fixed in the corresponding space[.
Generally speaking, covalent binding will lead to a strong and stable binding between the enzyme molecule and the carrier, and at the same time, the original conformation of the enzyme molecule will reduce the activity due to the influence of covalent binding. Although the covalent immobilization method is used, the activity of general enzymes is usually reduced after immobilization, and lipase is often an exception. One possible explanation is the unique structure of lipase, whose active site is often covered by a "lid"-like structure. Under the action of interface activation, the lipase molecular conformation changes and the lid opens, the active site is exposed and reacts with the substrate.
(2) Non-covalent binding method
The non-covalent binding between enzyme molecules and membranes to prepare immobilized enzyme membranes mostly involves hydrophobic, hydrophilic and electrostatic interactions. Among them, adsorption is a simple and common way. The adsorption is mainly attributed to the change of van der Waals force, entropy value and hydrogen bond generated by hydrophobic and hydrophilic interactions when enzyme molecules and membranes are in contact. For most membranes, in order to achieve better immobilization effect and better enzyme activity, it is often necessary to modify the membrane. Adsorption can be performed on modified membranes. For example, lipases are generally more active at hydrophobic interfaces. In a study, it was found that chitosan membrane was grafted with stearic acid to increase its hydrophobicity, and the best grafting degree was about 30%. At this time, compared with untreated chitosan membrane, the enzyme Membrane activity increased by 25%. It is worth mentioning that the suitable enzymatic activity can only be obtained when the grafting degree is 30%, which means that the strong hydrophobic membrane does not necessarily lead to a highly active biocatalytic membrane. This is due to the fact that the hydrophobicity of the membrane reduces the biocompatibility. In addition, the strongly hydrophobic membrane has a high load of enzymes. Such a high load can easily lead to accumulation between enzyme molecules, increasing the contact space between the active site and the substrate. Steric hindrance, and then manifested as a decrease in enzyme activity. Although the force between the enzyme molecule and the membrane is relatively weak during adsorption and immobilization, the stability of the enzyme molecule can be enhanced by changing and optimizing the immobilization process and conditions. The preparation process of non-covalent binding immobilized enzymes is easier than the covalent binding process, however, the weak binding force between enzyme molecules and carriers makes the loss of enzyme molecules from the carrier relatively prominent. In order to reduce the loss of enzyme molecules, some researchers also added glutaraldehyde for cross-linking treatment.

(3) Embedding method
For most enzymes, enzyme embedding has the advantages of a wide range of applications and relatively little impact on the structure and function of the enzyme after immobilization, as shown in Table 1-5. There are generally two ways to embed enzymes in membranes. The first method is to mix the enzyme solution and the membrane solution to complete the enzyme embedding during the membrane making process; the second method is to embed the enzyme in the membrane pores by filtering the enzyme solution. In contrast, the residual enzyme activity of the latter was slightly higher than that of the former. Generally, cross-linking agents are rarely used for embedding. However, there are exceptions, such as Moradzadegan A et al. used the cross-linking agent glutaraldehyde to cross-link the fibers used for film formation, which also limited the covalent binding between enzyme molecules and the film. Goto M et al. carried out the cross-linking of enzyme molecules in the membrane pores to enhance their stability. However, the embedding method is easy to cover the active site of the enzyme molecule, increasing the steric hindrance between it and the substrate, which is not conducive to the normal performance of the activity.
Table 1-5 List of immobilized enzyme membranes by embedding method
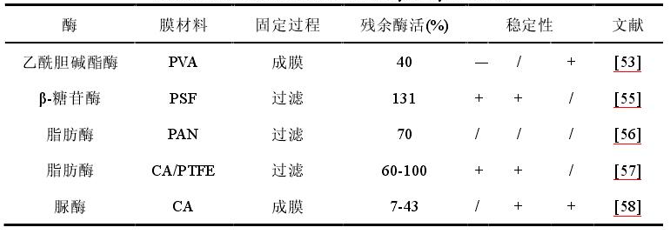
Note: Membrane formation refers to the mixing of enzyme solution and membrane solution to form a membrane, and filtration refers to the retention of enzyme solution by membrane filtration. "+" indicates an increase compared with the free enzyme, "-" indicates a decrease compared with the free enzyme, "→" indicates no change, and "/" indicates no relevant data.
Generally speaking, after the enzyme is immobilized, the original conformation of the enzyme molecule and its microenvironment often change, and the properties of the enzyme also change to varying degrees. Although the enzyme immobilization technology is divided into covalent, non-covalent and embedding, there is no clear method to detect which is the better immobilization technology. In contrast, the reduction of enzyme activity after covalent binding is more common. At the same time, the highest residual enzyme activity still came from the covalent binding of the enzyme on the membrane, which indicated that the covalent binding method had a stronger effect on the enzyme activity than the other two immobilization techniques. In a word, choosing a suitable immobilization method needs to fully consider the properties of the carrier material and the structure of the enzyme molecule itself, so as to avoid the destruction of the high-order conformation of the enzyme active center during the immobilization process.
This article is excerpted from "Application Research of PVA Composite Enzyme Membrane Reactor in the Synthesis of Lauryl Stearate " . The copyright belongs to the original author. If there is any infringement, please contact us immediately, and we will deal with it in time!
- 1How Flat Film Applicators Can Build a New Generation of Functional Materials on Fabrics
- 2NVP anode film FAQ and solution
- 3Application of laboratory coater in LED fluorescent film Spreader
- 4Application Technology of Laboratory Film Applicator in Dielectric Thin Film
- 5Comparison of Advantages and Disadvantages of Three Heating modes for Laboratory Film Applicators
- 6Application and selection of laboratory coater in PDMS thin film prepative
- 7Scraping machine selection case: film substrate large size high accuracy scraping solution
- 8Application of Film Applicator in PEM Research and Preparation
- 9Application of Film Applicator in PVDF-HFP Material Research








