What is a perovskite material?
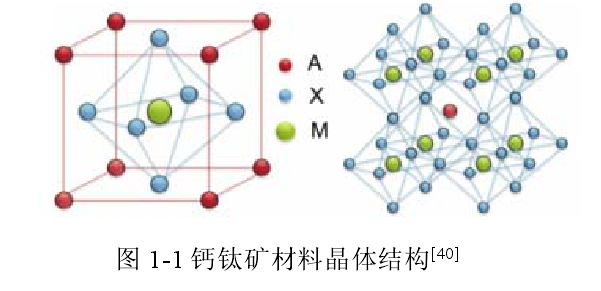
Perovskite materials refer to a class of materials that have the same crystal structure as CaTiO3, and the general chemical formula of its crystal structure is AMX3. The X element is an anion, and the A and M ions are cations of different sizes. The cation M coordinates with the anion X to form a regular octahedral symmetrical structure, and the cation A is located in the gap of the regular octahedron, and the M ion is located in the center of the regular octahedron. The ions form a cubic symmetric structure, thus forming a three-dimensional network periodic structure as shown in Figure 1-1.
Organic-inorganic hybrid perovskite materials are the core materials of PSCs, in which X elements are generally Cl, Br, or I and other halogen elements, M elements are generally Pb or Sn, etc., and A elements - are generally CH3NH3+ or HN=CH(NH3)+ etc. [41]. Due to the existence of organic molecules in the structure, and organic molecules have flexible orientation , the crystal does not have the symmetry in the crystal structure of inorganic perovskite materials, resulting in a decrease in the symmetry of the crystal structure of organic-inorganic hybrid perovskite materials . That is to say, since the orientation of organic molecules in the hybrid perovskite material crystal is random, the structure of the hybrid perovskite material crystal no longer has the symmetry of space inversion.
The stability of the hybrid perovskite crystal structure varies with the constituent elements. Taking CH3NH;PbI3 as an example, in this structure, the atomic bonds of PbI2 in the two-dimensional layered structure are changed , forming a three-dimensional CH3NH3PbI3 structure, which brings excellent properties, such as high absorption rate, long exciton lifetime, diffusion length, high ambipolar mobility, etc. [42. Theoretically, the use of cations with a relatively large ionic radius will expand the hybrid perovskite lattice, narrow the band gap, and red-shift the absorption spectrum, which is very beneficial to obtain a higher . However, too large cation radius will destroy the stability , thereby affecting its photoelectric performance. In addition, the change plays a very important role in adjusting the band gap of the hybrid perovskite material, so changing the metal cation Pb2+ will also change the hybrid perovskite Material crystal stability. Also for halide anions, as the halide ion radius increases, the lattice constant of the hybrid perovskite increases, so adjusting the use will also affect the crystal structure of the hybrid perovskite material [43]. At the same time, the study found that changes in temperature and air environment can also lead to changes in the stability of hybrid perovskite crystals.
When preparing the hybrid perovskite layer in PSCs, there is usually a heating process of sintering annealing. On the one hand, this step is to remove the organic solvent in the film, and on the other hand, it is to accelerate the crystallization of the hybrid perovskite crystal , but too high a temperature will decompose the hybrid perovskite material to form PbI2, CH3NH2 and HI44] . Zhang Danfei et al. explored the factors affecting the stability of hybrid perovskite solar cells and found that: the perovskite light-absorbing layer was placed in a humidity of 65% and 35 "C light environment for 18 hours, and the absorption significantly weakened. The reason is the decomposition of perovskite materials[45]. In addition, hybrid perovskite materials are also very sensitive to moisture and oxygen in the air environment , this material will generate PbI2 and CH3NH3I under the influence of H2O, and CH3NH3I will Further decompose into CH3NH2 and HI, and HI will react with oxygen in the air to generate I2 and H2O[46].The above analysis shows that the crystal structure of hybrid perovskite materials is very easy to change, and directly affects the hybridization The performance of PSCs and the atmospheric environment also have a great influence on the stability of hybrid perovskite material crystals. These are also the focus and hotspots of research by researchers at home and abroad.



