Corrosion Assessment: 8 Corrosion Tests to Help Engineers Mitigate Corrosion

Corrosion testing is one of the very important duties of a corrosion engineer. In fact, mitigating or eliminating corrosion in any industry is nearly impossible without a corrosion assessment.
Corrosion inspections are performed for several reasons. Sometimes, during the material selection process for industrial applications, it is necessary to evaluate different kinds of materials in a specific environment. Evaluating a new alloy in different types of environments for comparison to conventional commercial alloys; evaluating the effectiveness of inhibitors in reducing the corrosion rate of metals; and understanding corrosion mechanisms are other reasons.
Corrosion tests are usually divided into two categories: laboratory tests and field tests, each with its own advantages and disadvantages. For example, the environmental conditions in real applications are different from those in a laboratory environment. Therefore, it is difficult to extrapolate the results of laboratory tests to an industrial setting. On the other hand, in laboratory tests, the corrosiveness of the environment can be accelerated to obtain faster results, which is not possible in field tests.
Here, we'll learn about eight key corrosion tests that corrosion engineers use to help understand and mitigate corrosion problems in the field.
Laboratory Corrosion Testing
immersion test
One of the common and simple methods in laboratory testing is the immersion test. In this test, the procedure of which is spelled out by ASTM and NACE, the weight of a dry test sample is measured by an analytical balance before and after exposure to a corrosive environment for a specified time. Before and after weighing samples, specific preparations should be performed to remove any corrosion products or organic contamination. Corrosion rates for corrosion-resistant samples are usually calculated as weight loss or thickness loss in mils (0.001 inch) per year (mpy) or millimeters per year (mm/yr). Results depend on the type of metal tested (specific gravity), exposed surface area and test duration factors.
visual inspection
Some visual inspection is also recommended to assess localized corrosion such as pitting or spalling. In addition, optical or scanning electron microscopy; elemental and compositional analysis such as energy dispersive X-ray spectroscopy (EDX); X-ray diffraction (XRD); and energy dispersive X-ray spectroscopy (XPS) are more accurate methods for evaluating corroded surfaces and corrosion products useful technology.
There are several ways to evaluate test samples for pitting. Determining the density of pits (the number of pits in a specific surface area) or the pitting coefficient (the ratio of the depth of the deepest pit divided by the value of thickness loss due to uniform corrosion) are two important methods for evaluating pitting corrosion. There are different types of utilities to measure pit depth. When a dimple gauge is not available, a profile gauge can be used to obtain a profile of the dimple depth.
Salt Spray/Fog Test
Some test samples and procedures are designed to evaluate specific types of corrosion such as crevice corrosion, stress corrosion cracking and erosion corrosion. Atmospheric corrosion of coated samples can be checked by salt spray test or fog test. Here, a 5% NaCl solution is nebulized in a chamber temperature regulated to 95°F (35°C). The time a sample can resist corrosion is the criterion used to understand the durability of the test sample. Although the environment in the salt spray test was an accelerated marine atmosphere, it is generally accepted that the results of the salt spray test can be extrapolated to other atmospheric environments. (For more information on corrosion in marine environments, see Marine Structures Present Unique Challenges for Third-Party Inspectors.)
Aging test
In another atmospheric method called weathering testing, organic, paint-coated samples are examined for durability by exposing them to ultraviolet light and cyclic cooling and heating as well as corrosive environments.
Electrochemical test
Electrochemical testing is another type of laboratory test that can provide valuable information about the electrochemical reactions of corrosion and the mechanisms behind them. A potentiostat is commonly used to perform such tests. Typically a three-electrode setup is used, consisting of a working electrode, a reference electrode and a counter (auxiliary) electrode. Potential, current and time are three important parameters in electrochemical testing. In these tests, the applied potential is typically swept over a range and the current is measured.
There are several types of electrochemical corrosion tests. Each type serves a specific purpose.
Linear Polarization Resistance (LPR): A simple electrochemical corrosion test is linear polarization resistance, when the applied potential is in a narrow range (~20 mV ) while sweeping, the current is measured. The slope of the current versus potential curve represents the polarization resistance, which is inversely proportional to the corrosion rate. This test is very quick and straightforward and is generally considered a non-destructive test. Furthermore, this method is very useful for measuring extremely low corrosion rates. This is important in some industrial systems, such as food processing, nuclear power, and pharmaceutical equipment.
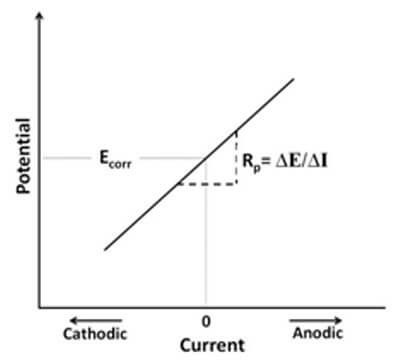
This graph shows the LPR curve. The slope of the line represents the polarization resistance (Rp).
Potentiodynamic Polarization Test: The passivation behavior of active-passive metals such as stainless steel can be assessed by potentiodynamic polarization testing. In this approach, the potential scan range is wide. The critical current density, passive potential and passive current density can be extracted from this test.
循环极化法:这是另一种用于确定主动-被动金属局部缝隙或点蚀倾向的测试。在该测试中,施加电位的扫描方向在跨无源区的某个电位处反转。前向和后向扫描的交叉点显示了局部腐蚀的趋势和强度。
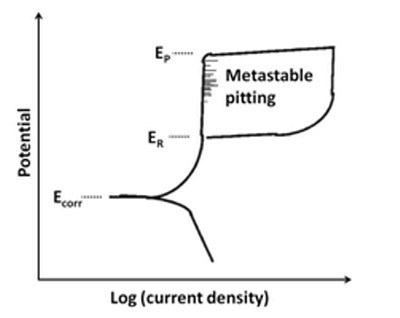
该图显示了用于评估点蚀的循环极化曲线。较小的E R和较大的亚稳态点蚀回路显示出对点蚀更敏感。
电化学动势再活化 (EPR) 测试:这是另一个建议用于预测不锈钢致敏化或晶间腐蚀趋势的测试。与 ASTM A-262 建议的其他常规晶间腐蚀测试(例如Huey或Streicher )相比,电化学动电位再活化非常简单和快速。
上述电化学测试是在直流条件下进行的。然而,了解亥姆霍兹双层(可以充当电容)或金属表面上的抑制剂吸附(可以充当电感)需要交流电流。这种类型的测试称为电化学阻抗谱 (EIS),可以揭示有关腐蚀机制的有价值信息。此外,当电化学系统中的整体电阻非常高时,例如当样品被厚有机涂层覆盖或浸入有机溶液中时,该技术非常有用。
现场腐蚀测试
腐蚀挂片
安装腐蚀试片是监测管道、热交换器和储罐腐蚀的一种非常简单且常用的方法。将优惠券插入带有优惠券支架的工厂或设备中一段时间。(在腐蚀挂片持有人如何保护您的宝贵资产中了解有关优惠券持有人的更多信息 .) 虽然许多因素会影响试片安装的位置,但试片通常放置在预计会出现严重腐蚀的位置。取回优惠券后,将考虑重量和尺寸的变化或目视检查。这种方法的缺点是不可能通过加速环境条件来获得更快的结果。
超声波厚度监测
超声波测厚仪(UT)是用于监测管道或储罐内部腐蚀的仪器之一。由超声波换能器产生的超声波穿过后壁并反射回声源,从而可以通过测量反射时间并考虑声波在被测材料中的速度来计算金属的厚度. 当无法接触到测试样本的两侧时,UT 量规测试很有用。(有关超声波检测的更多信息,请参见工艺管道的 CUI 检测技术(第2部分)。)
电阻测试
Electrical resistance (ER) probes are used to measure the corrosion rate of a specimen, especially when in-line corrosion rates are required. When a metal corrodes in the environment, its electrical resistance increases due to a decrease in the thickness or surface area of the cross-section. By measuring the change in metal resistance over time, the rate at which the metal dissolves can be determined and the corrosion rate calculated in mpy or mm/yr. ER probes can be used in any type of environment including aqueous solutions, oils (hydrocarbons), soils, gases and atmospheres. Probes can be made in various geometries depending on the type of metal, the system and the environment being measured.
There are many other tests that can be used to monitor structures protected from corrosion through cathodic protection. Most of these tests are based on the measurement of the electrochemical potential of structures and environments.
- 1Cyclic corrosion test and its test procedures
- 2The principle and application of salt spray corrosion Test Chamber, the operation and selection method
- 3What are the artificial simulated salt spray corrosion test methods?
- 4Cyclic Corrosion Testing and Applicable Standards
- 5Corrosion test: salt spray test + humidity test
- 68 corrosion tests
Mehdi Yari
- 7Principle of cyclic corrosion test
- 8Corrosion Test Types for Plated Parts and Assemblies
- 9What is Automotive Cyclic Corrosion Test CCT?
Mark
-
HZAOC LSO2-100 SO2 corrosion Test Chamber$ 15123.00
-
HZAOC SO2-300 SO2 corrosion Test Chamber$ 15358.00
-
HZAOC SO2-250 SO2 corrosion Test Chamber$ 15358.00




