What are acid copolymers and ionomers?
Acid copolymers and ionomers are high performance resins with significantly better adhesion, heat seal and barrier properties than conventional polyethylene. Figure 21.1 shows the structures of acrylic and methacrylic acid copolymers, two acid copolymers currently commercially available in the United States. The presence of methyl pendant groups in methacrylic acid copolymers leads to some slight differences between the two resin types, but they can be considered equivalent after accounting for the molecular weight difference of the comonomers. For example, 10% by weight of an acrylic acid copolymer has a carboxyl content equivalent to 12% by weight of a methacrylic acid copolymer.
Ionomers (Fig. 21.2) are derived from acidic copolymers by partially neutralizing the carboxyl groups with sodium or zinc ions. Due to the large increase in melt viscosity caused by the neutralization reaction, it is often called ionic crosslinking.
As shown in Figure 21.3, in acidic copolymers and ionomers, both melt and solid-state properties are strongly influenced by intramolecular hydrogen bonding. The forces involved in hydrogen bonding are almost 10 times greater than the intramolecular forces in nonpolar polyethylene.
Ionomers are characterized by a combination of hydrogen bonds and interchain ionic forces, possibly an order of magnitude stronger than hydrogen bonds. As a result, ionomers exhibit a broad range of melt and solid-state properties, including better hot tack and grease resistance than acid copolymers of equivalent acid content.
For acid copolymers, melt index and acid content are the main variables available to the resin manufacturer. Because melt index is a measure of melt viscosity, it is primarily related to the processing characteristics of the resin. Resins used today for extrusion coating applications are in the melt index range of 5 to 15 to accommodate a wide range of processing needs.
Resins with acid contents ranging from 3% to 15% are currently on the market. The effect of increasing the acid content is as follows:
• Better foil adhesion
• Better hot tack
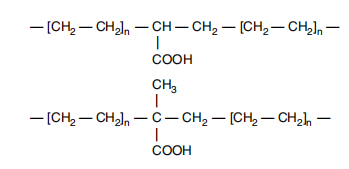
Figure 21.1 Structure of ethylene acrylic acid (top) and methacrylic acid (bottom) copolymers.

Figure 21.2 Ionomer structure.
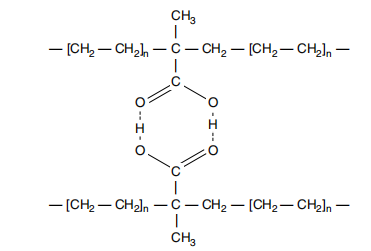
Figure 21.3 Intramolecular hydrogen bonding.
• Lower seal initiation temperature
• Better oil resistance
• better wear resistance
• Higher air and moisture permeability
The important effect is improved adhesion to unprimed aluminum foil, since most commercial applications of acid copolymers involve adhesion to aluminum foil.
Ionomers are characterized by additional variables that can be manipulated to obtain a wider range of properties in the final product. These variables include:
• Melt index (molecular weight) of the base resin
• Percentage of acid in base resin
• Degree of ionic crosslinking (acid neutralization percentage)
• Type of ion used for neutralization (Na or Zn)
In particular, the use of sodium and zinc ions produced a family of resins with distinctly different properties. Zinc ionomers are used in most extrusion coating applications due to their excellent adhesion to unprimed foils.
The effect of composition on ionomer properties is shown in Table 21.1. In general, ionomers based on high acid copolymers with a high degree of neutralization have better heat seal and product resistance properties. However, these advantages are balanced by the need for relatively high free carboxyl content for better foil adhesion.
In practice, most extrusion coating resins used for coextrusion with nylon have a high degree of neutralization. Sodium ionomers have limited use in extrusion coating applications where maximum grease resistance is required, but these are on paper or primed foils.
-
-
-
JINGWEN JW-RF330SW Heat Sealing Tester$ 2201.00


