What is the electrolysis process?
The electrolytic process uniformly deposits a dense high-chromium non-magnetic alloy on the surface to be treated basic metal. The alloys used for electrolysis provide an unusual combination of bearing properties: extraordinary wear resistance, very low coefficient of friction, smooth sliding properties, excellent anti-seizure properties and beneficial corrosion resistance. Plated parts perform better and last 10 times longer than untreated products.
All electrolysis facilities carefully monitor the solution and application process. The result is a fine-grained chrome coating that is very hard, thin and dense, with absolute bonding qualities. The electrolytic process deposits 99% of the chromium coating on the base metal surface, while conventional chrome plating processes tend to deposit 82% to 88% chromium normally in most applications.
Electrolysis requires the cleaning and removal of the matrix on the surface of the base metal through a variety of cleaning processes, using an improved electrocoating process, a solution that combines the chromium metal element with the pores of the base metal surface. It is during this process that Absolute produces the adhesive properties and qualities of electrolysis. Electrolytic coatings will not flake, crumble or flake off metal substrates when routine ASTM bend and impact tests are performed. After electrolysis of metal surfaces, there are always three basic factors:
• Increased wear (Rockwell superficial hardness 70 to 72 Rc)
• Increased lubricating properties
• Excellent corrosion resistance
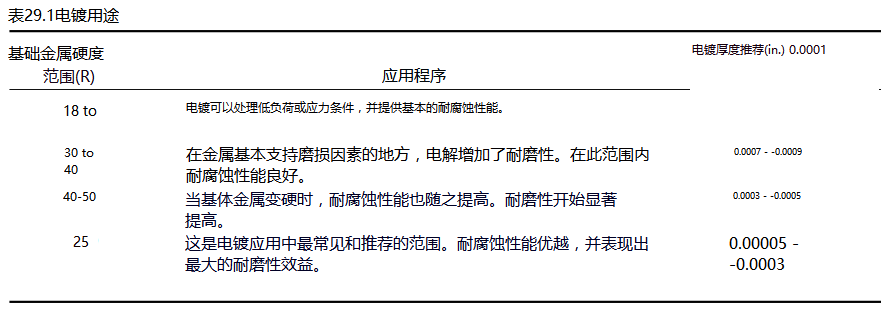
- 1Working principle and application of UV coating Cupping testing device
- 2Application of shakeout Tester in organic polymer film abrasion resistance testing
- 3Application of shakeout Tester in aluminum surface coating abrasion resistance testing
- 4Paint film abrasion resistance and test method thereof
- 5Polyethylene (PE) coating indentation hardness inspection and its importance in pipeline corrosion protection
- 6Paint film abrasion resistance and its test method - rubber abrasive wheels method
- 7Determination of abrasion resistance of paint film
- 8Determination method of paint film abrasion resistance and its importance
- 9Coating performance testing: the key to ensuring quality coatings
